Veeravalli Stroke and Spinal Cord Injury Lab
The Veeravalli lab focuses on explaining cellular and molecular mechanisms of tissue damage and functional recovery, identifying and validating novel targets, and evaluating the efficacy of novel treatments to reduce tissue damage and improve functional recovery after stroke and spinal cord injury.
Areas of Focus
The research team investigates the efficacy of novel treatments to:
- Reduce brain or spinal cord injury, inflammation, apoptosis, and demyelination
- Prevent blood-brain barrier or blood-spinal cord barrier disruption
- Preserve neurological function
- Promote the recovery of sensorimotor and cognitive function in rodent models of ischemic stroke and spinal cord injury.
The research goals are met through the use of multiple treatment strategies, including cell-based therapy with mesenchymal stem cells, target gene silencing therapy with siRNA/shRNA, and recombinant antibody therapy using single-chain variable fragment (scFv) antibodies.
Meet the Research Team
Collaborators
-
Raghu Vemuganti, PhD
Professor and Vice-Chair of Neurovascular Surgery
University of Wisconsin-Madison -
Adinarayana Kunamneni, PhD
Assistant Professor
Mayo Clinic Jacksonville -
Sourabh Lahoti, MD
Clinical Assistant Professor of Neurology, UICOMP
Neurointerventional Surgeon and Vascular Neurologist
Illinois Neurological Institute, OSF HealthCare
Stroke Projects
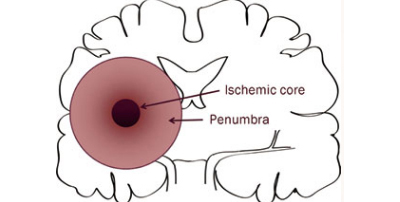
Stroke is the fifth leading cause of death and a leading cause of disability in the United States. Globally, fifteen million people suffer from a stroke each year and five million stroke patients die with another five million left permanently disabled. Despite decades of research, no clinically effective pharmacotherapies exist to facilitate cellular functional recovery after a stroke. It is still an unmet medical need. None of the treatment options thus far has proven efficacious in clinical studies, despite tremendous outcome in preclinical studies. The failure of many agents in clinical trials could be due to the complex pathology of ischemic stroke and illustrates the sobering challenge ahead for translational stroke therapy. In this scenario, it is very important to target several key molecules simultaneously, which can work by multiple mechanisms to control the early and delayed brain injury in both the ischemic core and the penumbra. The novel approach should adopt preclinical testing, advance the understanding of the pathophysiology of stroke and make it possible to translate it from bench to bedside.
Stem cell transplantation and gene silencing
Our research targets both the core and the penumbra by a combination of two cutting edge approaches; stem cell transplantation and target gene silencing, which we believe could significantly improve the microenvironment of ischemic brain as well as functional recovery after ischemic stroke.
Our lab works with both rat and mice stroke models. Stroke is a sexually dimorphic disease. While, young females are protected against ischemia compared to males (partially due to the protective effect of estrogens), older females exhibit extensive brain damage and poor recovery. Our experimental approach is rigorous as we use both males and females, young and older animals, and test the key outcomes in other stroke labs.
More info about stroke projects
Stroke research
- Challa SR, Nalamolu KR, Fornal CA, Mohandass A, Mussman JP, Schaibley C, Kashyap A, Sama V, Wang BC, Klopfenstein JD, Pinson DM, Kunamneni A, Veeravalli KK. The interplay between MMP-12 and t-PA in the brain after ischemic stroke. Neurochemistry International 2022; 161: 105436.
- Challa SR, Nalamolu KR, Fornal CA, Wang BC, Martin RC, Olson EA, Ujjainwala AL, Pinson DM, Klopfenstein JD, Veeravalli KK. Therapeutic efficacy of MMP-12 suppression on neurological recovery after ischemic stroke: optimal treatment timing and duration. Frontiers in Neuroscience 2022; 16: 1012812.
- Nalamolu KR, Chelluboina B, Fornal CA, Challa SR, Pinson DM, Wang DZ, Klopfenstein JD, Veeravalli KK. Stem cell treatment improves post-stroke neurological outcomes: A comparative study in male and female rats. Stroke and Vascular Neurology 2021; 6: 519-527.
- Nalamolu KR, Challa SR, Fornal CA, Grudzien NA, Jorgenson LC, Choudry MM, Smith NJ, Palmer CJ, Pinson DM, Klopfenstein JD, Veeravalli KK. Attenuation of the induction of TLRs 2 and 4 mitigates inflammation and promotes neurological recovery after focal cerebral ischemia. Translational Stroke Research 2021; 12: 923-936.
- Nalamolu KR, Venkatesh I, Mohandass A, Klopfenstein JD, Pinson DM, Wang DZ, Kunamneni A, Veeravalli KK. Exosomes secreted by the cocultures of normal and oxygen-glucose deprived stem cells improve post-stroke outcome. NeuroMolecular Medicine 2019; 21: 529-539.
- Nalamolu KR, Venkatesh I, Mohandass A, Klopfenstein JD, Pinson DM, Wang DZ, Veeravalli KK. Exosomes treatment mitigates ischemic brain damage but does not improve post-stroke neurological outcome. Cell Physiol and Biochem 2019; 52: 1280-1291.
- Nalamolu KR, Smith NJ, Chelluboina B, Klopfenstein JD, Pinson DM, Wang DZ, Vemuganti R, Veeravalli KK. Prevention of the severity of post-ischemic inflammation and brain damage by simultaneous knockdown of toll-like receptors 2 and 4. Neuroscience 2018; 373: 82-91.
- Chelluboina B, Klopfenstein JD, Pinson DM, Wang DZ, Vemuganti R, Veeravalli KK. Matrix metalloproteinase-12 induces blood-brain barrier damage after focal cerebral ischemia. Stroke 2015; 46: 3523-31.
- Chelluboina B, Warhekar A, Dillard M, Klopfenstein JD, Pinson DM, Wang DZ, Veeravalli KK. Post-transcriptional inactivation of matrix metalloproteinase-12 after focal cerebral ischemia attenuates brain damage. Scientific Reports 2015; 5: 9504.
- Chelluboina B, Veeravalli KK. Application of human umbilical cord blood-derived mononuclear cells in animal models of ischemic stroke. Journal of Stem Cell Research and Transplantation 2015; 2: 1014.
- Chelluboina B, Klopfenstein JD, Gujrati M, Rao JS, Veeravalli KK. Temporal regulation of apoptotic and anti-apoptotic molecules after middle cerebral artery occlusion followed by reperfusion. Molecular Neurobiology 2014; 49: 50-65.
- Chelluboina B, Klopfenstein JD, Pinson DM, Wang DZ, Veeravalli KK. Stem cell treatment after cerebral ischemia regulates the gene expression of apoptotic molecules. Neurochemical Research 2014; 39: 1511-1521.
Spinal Injury Projects
SCI is a devastating injury which involves an initial mechanical damage followed by a series of cellular and molecular secondary events resulting in the progressive destruction of spinal cord tissue. Neuropathic pain (NP) is one of the most debilitating sequelae of neurotrauma and remains an unmet clinical need for at least 40% of patients with SCI. Methylprednisolone is the only FDA approved drug that is currently available to limit the extent of SCI in the acute settings but it does nothing for prevention or mitigation of subsequent neuropathic pain following SCI. Despite decades of extensive research in this area, no clinically effective therapies exist to modulate neuropathic pain and facilitate functional recovery after spinal cord injury.
SCI results in a multitude of changes affecting several different cell types, leading to a complex pathological picture. Most research findings to date suggest that no single therapy will be sufficient to overcome the myriad of biological cascade initiated after SCI. Effective treatments of SCI require a multifaceted approach using a combination of different methodologies and therapeutic approaches over different time to address many of the devastating issues besides functional impairment such as chronic pain associated with SCI.
Stem cell transplantation and gene silencing
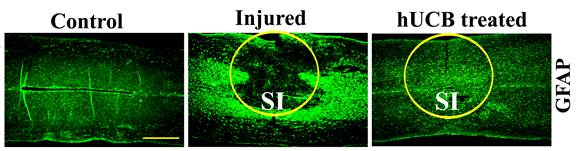
Our current research utilizes stem cell transplantation and gene silencing. A variety of different stem cell types have been evaluated in animal models and humans with SCI. Previous studies have reported that human umbilical cord blood-derived mesenchymal stem cells (hUCB-MSCs) promote neural repair after SCI, even when administered 5 days after injury. Transplanted hUCB-MSCs differentiate into various neural cells and induce motor function improvement in SCI rat models. In concert with these findings, we also have recently reported that hUCB-MSCs improved the locomotor recovery of spinal cord injured rats while regulating the expression of several genes related to apoptosis, axon outgrowth and myelin degradation. However, more detailed experiments are needed to delineate the mechanism of how hUCB-MSCs modulate NP and functional improvement after SCI.
More info about spinal injury projects
Spinal Cord Injury Research
Selected works
- Chelluboina B, Dinh DH, Veeravalli KK. Transdifferentiation of differentiated stem cells contributes to remyelination. Stem Cell Research & Therapy 2015; 6: 191.
- Dasari VR, Veeravalli KK, Dinh DH. Mesenchymal stem cells in the treatment of spinal cord injuries: A review. World Journal of Stem Cells 2014; 6: 120-133.
- Veeravalli KK, Dasari VR, Rao JS. Regulation of proteases after spinal cord injury. J Neurotrauma 2012; 29: 1-12
- Dasari VR, Veeravalli KK, Fassett D, Rao JS, Dinh DH. Mesenchymal stem cell therapy for apoptosis after spinal cord injury. Advanced Understanding of Neurodegenerative diseases ISBN 978-953-307-529-7, Intech (Publisher), 2011; 365-394.
- Kotipatruni RR, Dasari VR, Veeravalli KK, Dinh DH, Fassett D, Rao JS. p53- and Bax-mediated apoptosis in injured rat spinal cord. Neurochemical Research 2011; 36: 2063-2074.
Selected works continued
- Veeravalli KK, Dasari VR, Fassett D, Dinh DH, Rao JS. Human umbilical cord blood derived mesenchymal stem cells upregulate myelin basic protein in shiverer mice. Stem Cells and Development 2011; 20: 881-891.
- Veeravalli KK, Dasari VR, Tsung AJ, Dinh DH, Gujrati M, Fassett D, Rao JS. Human umbilical cord blood stem cells upregulate matrix metalloproteinase-2 in rats after spinal cord injury. Neurobiology of Disease 2009; 36: 200-212.
- Dasari VR, Veeravalli KK, Tsung AJ, Gondi CS, Gujrati M, Dinh DH, Rao JS. Neuronal apoptosis is inhibited by cord blood stem cells after spinal cord injury. J Neurotrauma 2009; 26: 2057-2069.
- Veeravalli KK, Dasari VR, Tsung AJ, Dinh DH, Gujrati M, Fassett D, Rao JS. Stem Cells downregulate the elevated levels of tissue plasminogen activator in rats after spinal cord injury. Neurochemical Research 2009; 34: 1183-1194.
- Dasari VR, Veeravalli KK, Saving KL, Gujrati M, Fassett D, Klopfenstein JD, Dinh DH, Rao JS. Neuroprotection by cord blood stem cells against glutamate-induced apoptosis is mediated by Akt pathway. Neurobiology of Disease 2008; 32(3): 486-498.







