Malchenko-Soares Lab
Research in our laboratory is focused on uncovering molecular pathways underlying intercellular mitochondrial transfer between Neural Stem Cells, Cancer Stem Cells and surrounding astrocytes. Intercellular mitochondrial transfer is a newly discovered unique communication system that utilizes formation of tunneling nanotubes for targeted mitochondrial transfer between donor and recipient cells
Overview Heading link
Research Expertise
- Generation of human induced Pluripotent Stem cells (hiPSCs), neuronal
differentiation of hiPSCs. - Differentiation of human neuronal stem cells into white matter specific astrocytes.
- Xenograft transplantation of human neuronal stem cells into specific brain areas of NOD-SCID mice.
- Generation and network analysis of large-scale mRNA and microRNA expression data, RNAseq, Exome.
Research Interests
- Neuronal differentiation of hiPSCs, particularly into Radial Glial cells
- Radial Glia differentiation into astrocytes
- Onset of brain tumor formation
- In-vivo brain tumor modeling
- Intercellular mitochondrial transfer
Induced Pluripotent Stem Cells Heading link
Overcoming ethical and technical barriers
Study of embryonic stem cell (ESC) differentiation is of enormous interest given the therapeutic potential in cell replacement therapy. However, there are well known ethical barriers related to the use of human embryos as the source of ESCs as well as “technical” ones related to generation of patient- specific ESCs. Induced pluripotent stem cell (iPSC) technology, which is based on reprogramming of differentiated somatic cells into a pluripotent state, could evade those barriers as iPSC can be derived with a specific desired genetic background, including patient-specific iPSC for disease models and for transplantation therapies, without the problems associated with immune rejection.
Using non-integrative Sendai viral vectors
Induction of pluripotency in adult human fibroblasts was originally achieved by Dr. Yamanaka and colleagues by enforced expression of four transcription factors: KLF4, c-MYC, OCT4, and SOX2, using retroviral vectors. Since we are generating the iPSC lines for the disease modeling, instead of the retroviral vectors, which integrate into genome during the reprogramming and potentially could cause undesirable gene alterations, we are using non-integrative Sendai viral vectors.
Radial Glial Cells Heading link
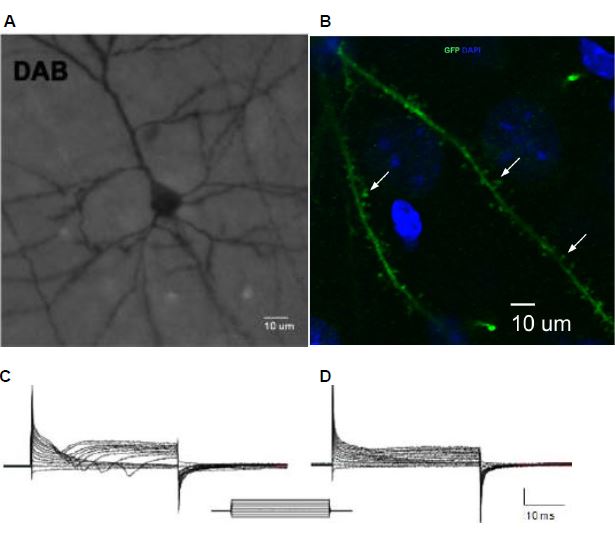
Barriers in studying nervous system disorders
The development and function of the nervous system is very complex and little understood. One of the biggest limitations in research of nervous system disorders is a lack of laboratory systems to study the course of disease and to identify potential drug targets. Human neuronal or brain cells typically cannot be examined experimentally until surgical or postmortem biopsy samples are obtained. These limitations become even more severe in the case of pediatric disorders.
Why study radial glial cells
Derivation of neuronal stem cells (NSC) can provide an efficient route to valuable disease models. radial glial (RG) cells are thought to be the progenitor cells for adult NSC, neurons, basal progenitors, astrocytes and oligodendrocytes, in addition to being responsible for the majority of neurogenesis in the developing brain. RG processes provide architectural support for the migration of newly generated neurons by forming scaffolds that span the central nervous system mantle region. In addition to being responsible for the majority of neurogenesis in the developing brain RG cells are not only of relevance to neurodegenerative diseases, and spinal injuries, but to brain tumors as well.
A new approach
Recently we reported a new approach that allowed us to derive large amounts of RG cells from hESC, and from hiPSC lines. We demonstrated that RG cells orthotopically transplanted to the motor cortex of 8-week old immunocompromised NOD-SCID mice can differentiate into functionally active, mature-appearing pyramidal and serotonergic neurons. The ability to generate these cells in amounts is very significant because it enables a wide range of experimentation that otherwise could not be done.
Publications Heading link
-
Selected publication
Sergey Malchenko, Jianping Xie, Maria de Fatima Bonaldo, Elio F. Vanin, Bula Bhattacharyya,Vasily Galat, William Goossens, Richard E.B. Seftor, John Crispino, Richard Miller, Martha C. Bohn, Mary J.C. Hendrix and Marcelo B. Soares.2014. Onset of rosette formation during spontaneous neural differentiation of hESC and hiPSC colonies. GENE 534 400-407.
In-Vivo Brain Tumor Modeling Heading link
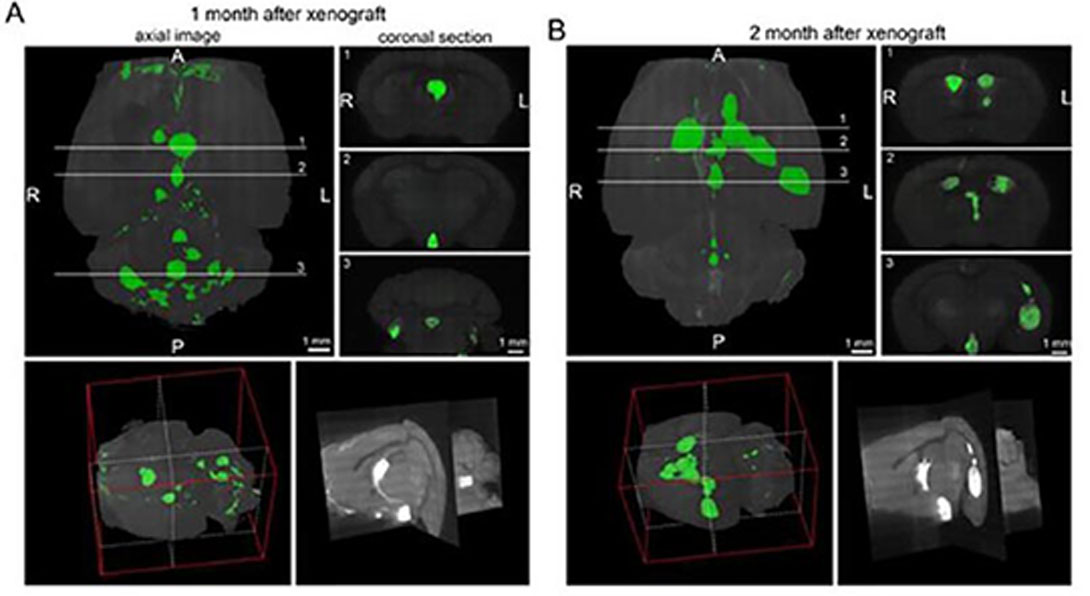
We also showed that orthotopic transplantations of the RG cells to the subventricular zone of the 3rd ventricle – but not to other transplantation sites – of the brain in immunocompromised NOD-SCID mice, gave rise to tumors that have the hallmarks of CNS primitive neuroectodermal tumors (PNETs). The resulting mouse model strikingly recapitulates the phenotype of PNETs. The tumors are highly invasive, and they effectively recruit mouse endothelial cells for angiogenesis.
The impact of this research
In addition to providing a prospect for drug screening and development of new therapeutic strategies, the resulting mouse model of PNETs offers an unprecedented opportunity to identify the cancer driving molecular alterations and the microenvironmental factors that are responsible for committing otherwise normal RG cells to a malignant phenotype.
Publications Heading link
-
selected publications
Sergey Malchenko, Simone Treiger Sredni, Atsushi Kasai, Kazuki Nagayasu, Kaoru Seiriki, Jianping Xie, Naira Margaryan, Hitoshi Hashimoto, Rishi Lulla, Lauren Pachman, Herbert Y. Meltzer, Mary J.C. Hendrix and Marcelo B. Soares. 2015. A Mouse Model of Radial Glial Cell-Derived Primitive Neuroectodermal Tumors. PLOS ONE Mar 31;10(3):e0121707. 3D
Sergey Malchenko, Simone Treiger Sredni, Yingtao Bi, Naira V. Margaryan,Jerusha Boyineni, Indra Mohanam, Tadanori Tomita, Ramana V. Davuluri, Marcelo B. Soares. 2017. Stabilization of HIF-1α and HIF-2α, upregulation of MYCC and accumulation of stabilized p53 constitute hallmarks of CNS-PNET animal model. PLOS ONE Mar 1;12(3):e0173106.
Malchenko S, Sredni ST, Boyineni J, Bi Y, Margaryan NV, Guda MR, Kostenko Y, Tomita T, Davuluri RV, Velpula K, Hendrix MJC, Soares MB. Characterization of brain tumor initiating cells isolated from an animal model of CNS primitive neuroectodermal tumors. Oncotarget. 2018 Feb 9;9(17):13733-13747.
Jerusha Boyineni, Simone T Sredni, Naira V Margaryan, Lusine Demirkhanyan, Michael Tye, Robert Johnson, Fernando Gonzalez-Nilo, Mary J C Hendrix, Evgeny Pavlov, Marcelo B Soares, Eleonora Zakharian, and Sergey Malchenko. Inorganic polyphosphate as an energy source in tumorigenesis. Oncotarget. 2020 Dec 15;11(50):4613-4624.
Intercellular mitochondrial transfer Heading link
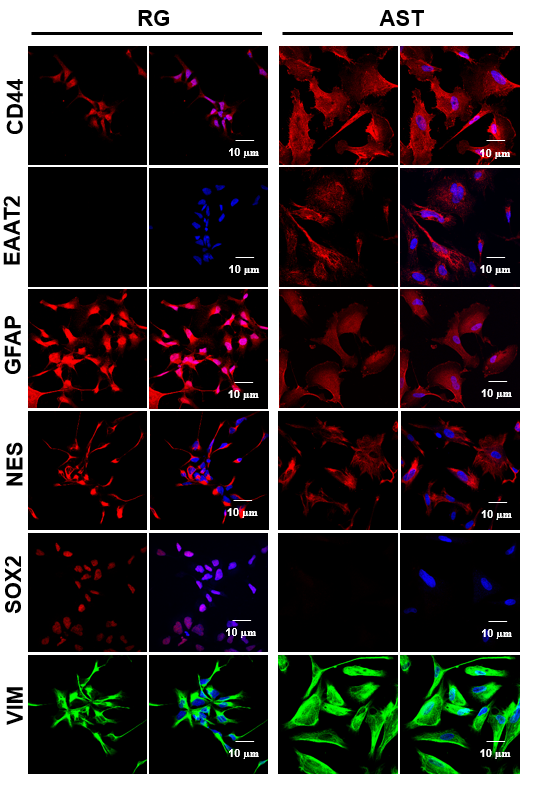
The communication between Neural Stem Cells (NSCs) and surrounding astrocytes is essential for the homeostasis of NSC niche. Intercellular mitochondrial transfer, a unique communication system that utilizes formation of tunneling nanotubes for targeted mitochondrial transfer between donor and recipient cells, has recently being identified in a wide range of cell types.
Intercellular mitochondrial transfer and cancer
Intercellular mitochondrial transfer has also been observed between different types of Cancer Stem cells (CSCs) and the neighboring cells, including brain CSCs and astrocytes. The CSC mitochondrial transfer significantly enhances overall tumor progression by reprogramming the neighboring cells. Despite the urgent need to investigate this newly identified phenomenon, the mitochondrial transfer in CNS remains largely uncharacterized.
Our work in this area
As the exact molecular pathways of the NSC and/or brain CSC mitochondria signaling that impact transcriptome of the recipient astrocytes remain to be decoded, we created a reproducible model utilizing large quantities of RG, BTICs and the astrocytes that were generated from the same induced pluripotent stem cells (iPSC). Currently, we are conducting different types of experimentations with this mode. These experiments involve epigenetics and metabolomics studies, which provide new insight into the mechanisms of mitochondrial retrograde signaling.
Video: Human Neural Stem Cells and Brain Tumor-Initiating Cells
Publications Heading link
-
selected publications
Jerusha Boyineni, Jason Michael Wood, Aditya Ravindra, Ethan Boley, Sarah E. Donohue, Marcelo Bento Soares and Sergey Malchenko. Prospective Approach to Decipher an Impact of Intercellular Mitochondrial Transfer from Human Neural Stem Cells and Brain Tumor Initiating Cells into the Neighboring Astrocytes. Cells 2024, 13(3), 204; https://doi.org/10.3390/cells13030204

